What is blister package for medicine (Part II)
Introduction
Blister packaging is portable, helps patients follow dosing regimens and protects drug quality over a longer shelf period. The advocate lists several aspects that blister packaging is superior to traditional packaging, including product integrity, production convenience, tamper-proof, reducing possibility of accidental misuse and good patient compliance. Materials used in blister packaging and compositions of typical blister packaging have been described earlier. This article will introduce machinery and assembly of blister packaging, cost of blister packaging and discuss the future trend of blister packaging.

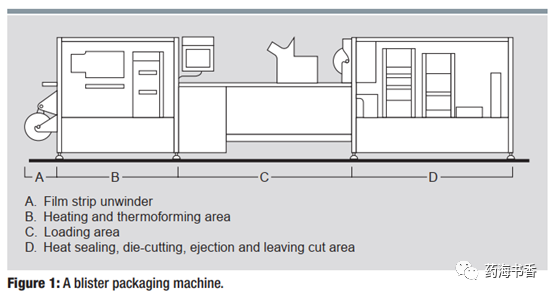
Modern thermoforming-filling-heat sealing blister packaging machine running speed can reach no more than 800 times/min. Today, the main focus on improving productivity is the application of microprocessor control, to connect filler and forming device with other downstream equipment such as cartoning machine and wrapping machine through electronic connections. The filler also fills tablet or solution into a single bubble to ensure accurate dosing. Modern machinery also uses integrated vision systems to help ensure the accuracy of blister filling and product integrity. These machines have become quite versatile and can be easily adapted to several different types of sealing foils and laminated hard sheets, allowing manufacturers to achieve better compatibility and patient compliance with drug and packaging materials. Blister packaging offers many advantages to pharmaceutical industry and the public, and packaging device will continue to support this proven packaging format. Improvements in molding methods, packaging materials and blister packaging machinery will be improving suitability of drug loading format and drug distribution. Figure 1 shows an example of a blister packaging device.
Conventional Assembly Process
Blister packaging machine conventional assembly process includes: heating plastic hard sheet, bubble thermoforming, tablet filling, sealing foil covering, and heat sealing. Its can be a simple manual process or a semi-automatic or fully automatic process. Also empty, pre-formed blisters and sealing foils can be purchased and then filled with separate drugs, while this process is not often. In contrast, it is more common that blister packaging completes molding and filling in one device (see Figure 2).
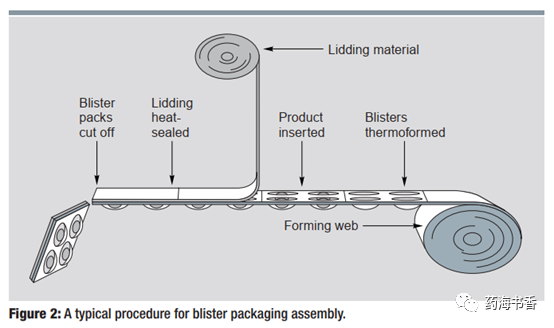
Assembly Details
Blister packaging machine is usually in intermittent movements. The sealing operation is done during the residence time required for thermoforming. Basic components and functions of intermittent packaging machine include the following aspects:
Ø Bearing shaft station
Bearing shaft station provides a rewinding speed for forming hard sheets and sealing foils that are adapted to operating speed of blister packaging machine (See Fig. 1, Part A).
Ø Heating station
Heating station raises the temperature of plastic molded hard sheet to a level suitable for stretching. PVC hard sheet can be heated to 120-140°C, PP hard sheet can be heated to 140-150°C. Composite aluminum foil does not require heating before forming (see Fig. 1, Part B).
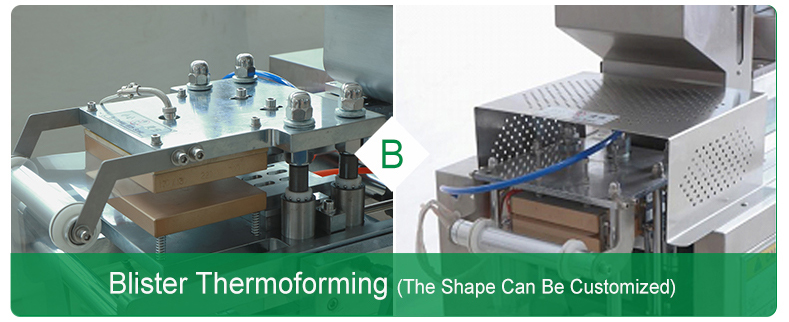
Ø Forming station
Forming station creates bubbles by compressing air or a formwork. Composite aluminum foil is stamped only with bubbles with a mechanical forming die (see Fig. 1, Part B).
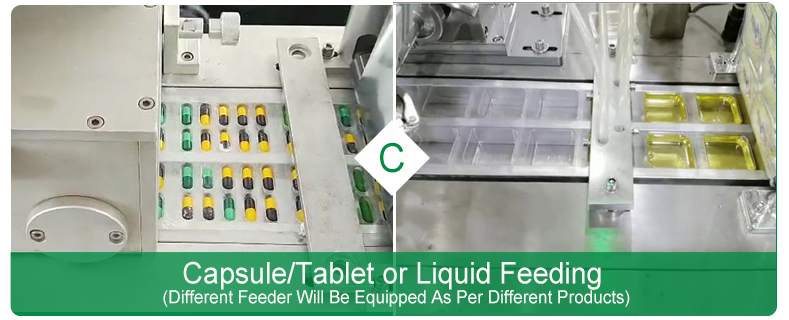
Ø Filling station
It fills product into the bubble. The filler can be interlocked, or simply sweep the product into the bubble (see Fig. 1, Part C).
Ø Sealing station
It seals the sealing foil on a molded hard sheet containing pharmaceutical product (see Fig. 1, Part D).
All heat sealing methods are made to pair sealing foil and hard sheet under constant pressure for a specified heating period. When heating is stopped, the fusion surface fuses and bonds almost instantaneously. Due to different machine types, sealing temperature is usually between 140 and 340°C.
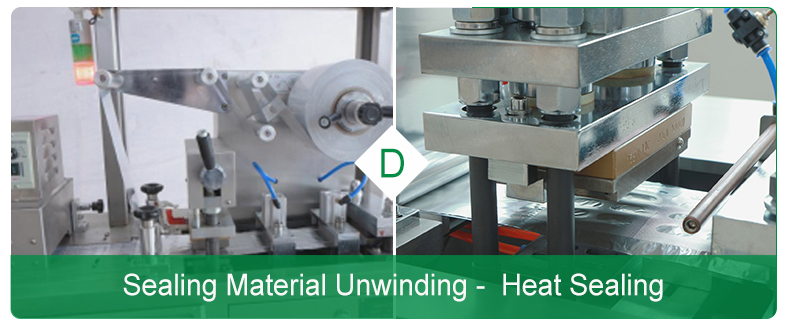
Ø Packaging and labeling
After packaging, it should place label and then print batch number. Embossing device makes a cross embossing at blister seal seam. Blanking station is divided into several plates, each plate usually contains 10 to 20 bubbles. Vision system inspects blister plate after filling for defects. And finally, the multifunctional packaging machine boxes and packs them.

Ø Blister packaging cost
Drug packaging has a significant impact on drug profitability, accounting for about 10% of packaging costs in prescription drugs and about 50% in over-the-counter drugs. Therefore, sales may be affected by positive or negative effects of packaging, especially OTC products.
Ø Cost comparison
The cost of various drug packages is rarely disclosed. However, one cost research reported that blisters versus bottles of oral medication per unit dose were cost-competitive. The report compares 60 cm³ and 125 cm³ bottles and 5 blisters with doses from 7 to 100 units, 6 blister structures (PVC, PVDC/PVC, PVC/Aclar for child protection and non-child protection). The researchers also considered cost of each component, including:
l Packaging line operation (e.g., equipment, speed, efficiency and labor) l
l Shipping l
l Freight l
l Distribution l
l Pharmacy inventory and dispensing
The research found that when considering the total cost of the system (including cost of refilling drugs and pharmacist time), blister packaging can represent an extremely economical traditional bottle. For example, a PVC blister with child protection can save $4.58 per 100 unit doses compared to a bottle. From viewpoint of production, bottle packaging tends to be more cost-effective than blister packaging, except for the most compact blister packaging and the simplest blister structure. Table 1 shows cost comparisons in the research.
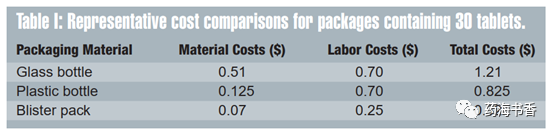
In this case, even if material cost is doubled, blister packaging design is still advantageous, because blister packaging material only accounts for a part of material cost, the rest is sealing foil. And the overall cost per packaging unit is $0.32. Compared with plastic or glass bottle packaging, labor cost savings are the advantages of flexible packaging such as blister because of its high degree of automation. Moreover, blister packaging is produced more economically since it is fully automatic and requires minimal labor support.
Ø Break-even point
In some product quantities, blister packaging has no advantage, since bottle packaging is more cost-effective. Generally, the break-even point is 100 dose units. If less than 100 tablets are sold, they can be packaged into the most economical blisters, that is, 10 tablets / plate, 10 plates. Drug distribution in larger quantities can be packaged into the most economical bottles. Therefore, the report shows that blisters are more cost-effective at 50-100 dose units and more expensive at greater than 100 dose units.
Ø Future trends
Unit dose packaging is a major trend in drug packaging, which has a great impact on blister packaging development. In addition, two major factors will have a huge impact on the development in the United States: clinical trials and regulatory developments.
l Clinical trials
As clinical trials increased, many product formulations become more complex, so more and more drug companies are turning to blister package. From perspectives of convenience and patient compliance, blister packaging used in clinical trials is beneficial. For example, in a dose-range research, patients were required to take 4 pills (or placebos) per day, blister is the simplest packing way. It is not very convenient for patients to take a tablet from one bottle first, and then take a tablet from another bottle. If pack them in a blister, all the drugs are in one place and easy to mark and distinguish.
l New regulations
Two regulatory regimes related to iron supplements and methamphetamine production will also influence the future development of blister package.
n Iron Supplements
The FDA Final Rule, titled "Iron-containing Supplements and Drugs: Labeling Warning Statements and Packaging Requirements per Unit Dose," went into effect on July 15, 1997. One of the clauses calls for at least 30 mg iron in a unit dose of blister packaging. Some companies had to remove their iron-containing products from shelves because they were not packaged in unit dose. Therefore, in order to comply with regulations, these companies will have to use blister package.
n Methamphetamine production
On October 3, 1996, President Clinton signed the Comprehensive Control of Methamphetamine Act. The Act expanded the control of certain chemicals used in methamphetamine production and increased penalties for trafficking and manufacture of methamphetamine and the listed chemicals, also broadened the control scope, including the sale of certain legally marketed products containing ephedrine, pseudoephedrine (PSE) and phenylpropanolamine (PPA). Matters involving PSE and PPA as main transactions need to be registered, recorded and reported in accordance with the requirements of the Act. However, the Act provided a safe harbor exemption for over-the-counter drugs containing PSE and PPA to be retailed as general goods. In order for a product to be included in the safe harbor, the following two requirements must be met:
- Packaging must contain no more than 3g of basic ingredients
- Product must be packed in blisters with no more than two pieces per blister (unless it is technically not possible to use blister, such as liquid preparations)
Since October 3, 1997, drugs not packaged under the Safe Harbor exemption must be registered with the Drug Administration, and the transactions must be kept in record if, for example, the single sale exceeds 24 g. In other words, to avoid registration process involved, retailers should sell certain over-the-counter drugs containing PSE and PPA in blister package. The purpose of the Act was to stop people from making illegal drugs out of these substances with impunity, making it harder to open each blister to get the required amount of drugs.
Conclusion
The demand for drug packaging is increasing and will continue as pharmaceutical companies become more reliant on packaging to protect and promote their products in the highly competitive and rapidly changing pharmaceutical market. While healthcare practitioners often choose pharmaceuticals, pharmaceutical manufacturers must design packaging with the user in mind. Just as appearance and ease of use are important for consumer products, they are also key to the success of a drug. In addition, for those over-the-counter drugs and nutritional supplements, consumer appeal is paramount. Companies that adopt blister packaging will undoubtedly face challenges and opportunities. The Consumer Product Safety Commission has asked packaging engineers to develop creative solutions to meet child protection and highly friendly requirements. With additional regulatory regimes such as ICH's testing guidelines and FDA regulations on iron supplements, the use of blister packaging and the application of innovative materials are expected to increase significantly.


 English
English



















