Background
Due to the widespread drug abuse problem in the United States and the inability to effectively crack down on and control numerous criminals involved in drug manufacturing and trafficking, relevant authorities are addressing the issue at the source.
Raw materials and production equipment used for drug manufacturing are now classified as controlled, restricted-sale products. Importing and locally producing/assembling such equipment (e.g., tablet presses, capsule filling machines) without a permit is prohibited.
What is a DEA License?
A DEA License (Drug Enforcement Administration) is a permit issued by the U.S. Drug Enforcement Administration (DEA), primarily used to regulate the manufacturing, distribution, and importation of controlled substances. This license is a mandatory qualification for legally producing or selling controlled substances, including narcotics, psychotropic drugs, etc.
Primary Purpose
The DEA license is mainly used to supervise the legal circulation of controlled substances, including narcotics, psychoactive substances, etc., ensuring their production, distribution, and importation comply with federal regulations.
Application Requirements
Applications must meet the following conditions:
Compliance Qualifications: Must complete the DEA registration process and submit necessary documents, such as business credentials, production plans, etc.
Security Standards: Must comply with federal security specifications for drug production, storage, and transportation.
Annual Review: License holders must submit annual operational reports and compliance certificates to the DEA.
Consequences of Non-Compliance
Producing or selling controlled substances without a license will result in penalties such as fines, suspension of business, etc., and is subject to strict oversight under the Controlled Substances Act.
Additional Clarification (Key Points)
The DEA license primarily targets the production and sale of controlled pharmaceuticals. However, currently, importing production equipment (such as tablet presses, capsule filling machines) intended for use with these substances also falls under DEA oversight to prevent illegal use. If you need to import such equipment, legitimate pharmaceutical companies, besides having FDA and EPA certifications, also require DEA permission (ensuring the equipment is not used to produce controlled drugs or, if used for producing controlled drugs, that it is properly controlled).
Other non-pharmaceutical companies currently only need to provide DEA certification. If your company already has FDA and EPA certifications, applying for a DEA license will be relatively straightforward.
Note:
Here we are discussing the one-time DEA license for importing pharmaceutical machinery, not the DEA license required by pharmacies or pharmaceutical manufacturers which requires annual review. Therefore, the registration process is not extremely difficult.
(The detailed application process will be provided later in the document.)
Reiteration
Without a DEA license, US businesses and individuals buyers cannot import tablet presses and capsule filling machines.
Alternative methods:
Smuggling - this is illegal.
Using strong connections and influence to get CBP to grant clearance (it's better to use those connections to obtain the DEA license legally).
Become a legitimate businessperson and conduct legal operations.
How To Fill Out Form 452 To Obtain A DEA License For Tableting & Encapsulating Machines Import
Step 1: Create Your DEA Online Account & Access Form 452
Visit the DEA Diversion Control Division website, log in to the Spring system. On the Controlled Machinery homepage, select code 452---the specific form for importing controlled machines. Click "Import" to begin your DEA License application process. Creating an online profile ensures you can revisit your application, save drafts, and download completed forms once the DEA License is granted.
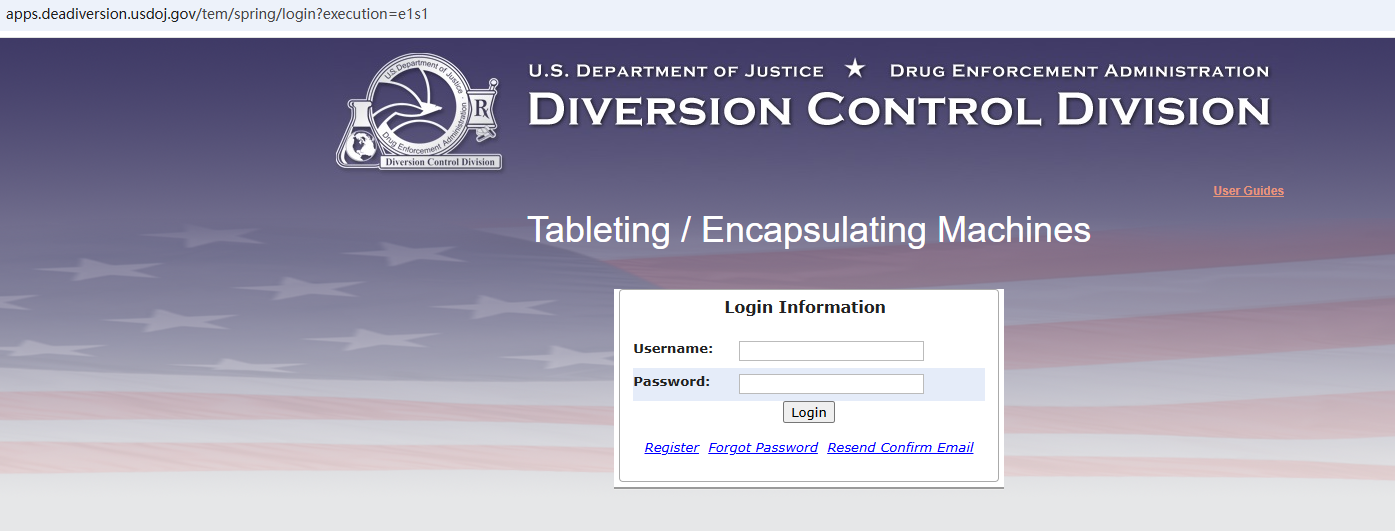
(1). Login page. Enter your username and password and click Login:
https://apps.deadiversion.usdoj.gov/tem/spring/login

If you haven't already registered: After you verify your email and set up multi-factor authentication, log in to the Spring system.
Usually select 'Company'
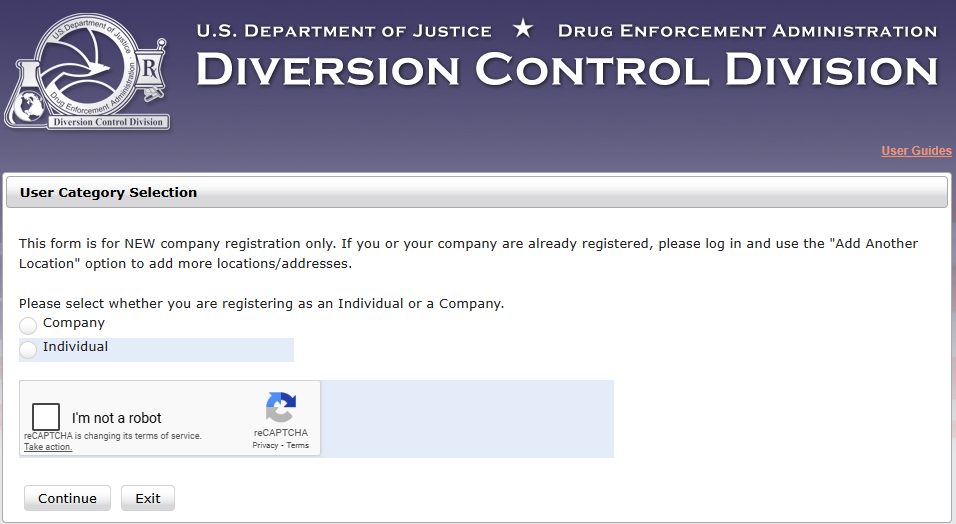
Click 'Next'
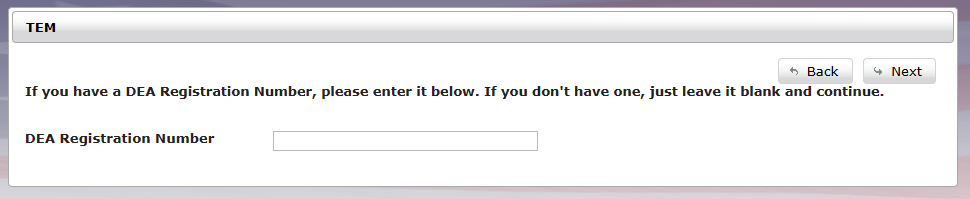
Enter your company information
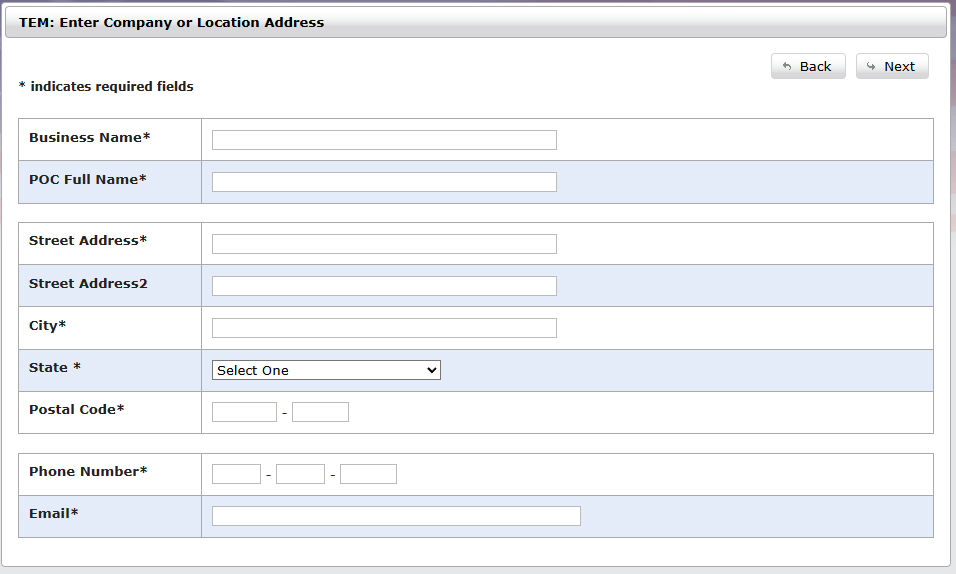
Verify your email within four hours and further complete your registration information.
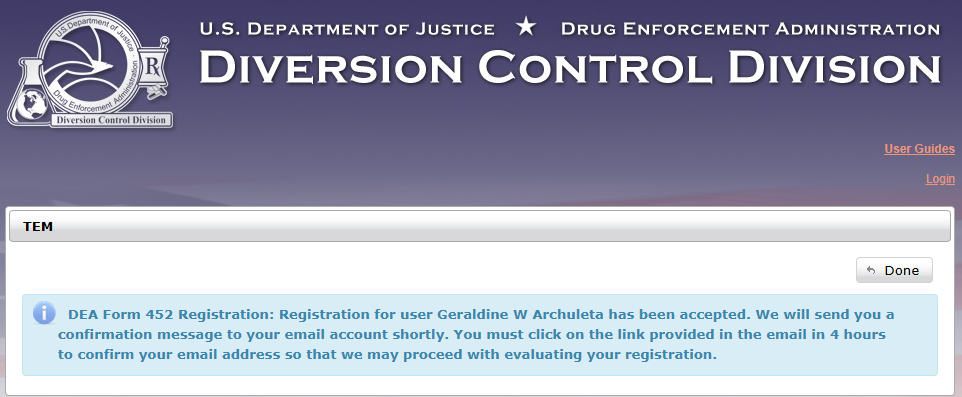
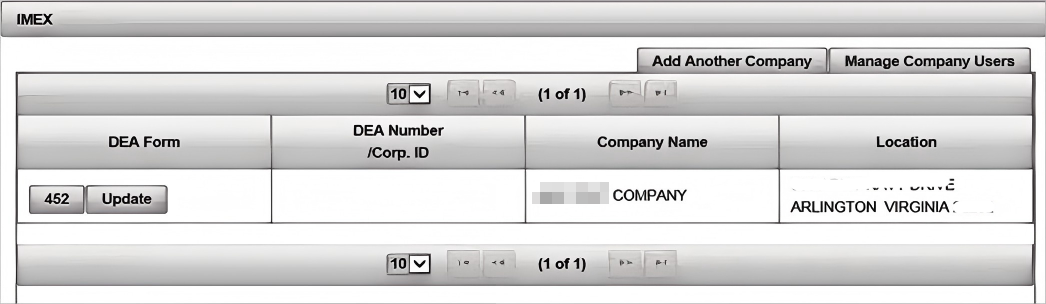
(2). Control machine home page. Click 452
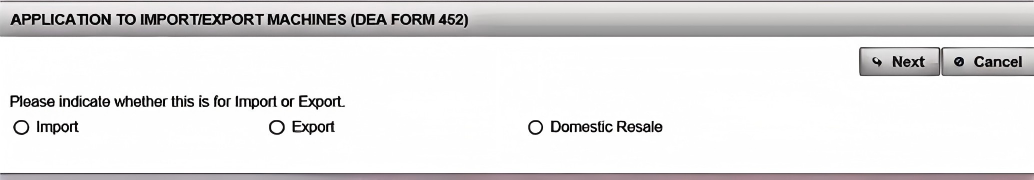
(3). Click "Import" to complete the "Application to Import Machinery" (DEA Form 452). Click "Next"
Step 2: Complete the Importer, Foreign Supplier, and Broker Details
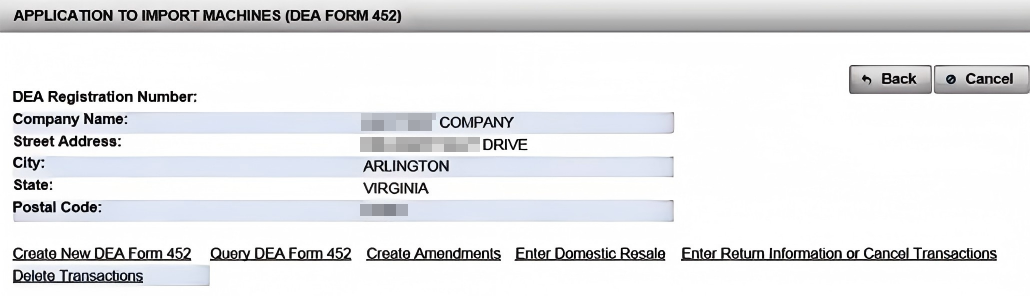
Once you click "Create New DEA Form 452," the system prompts you to add your company's information as the importer. Enter your legal business name, address, and contact details exactly as they appear on your corporate documents. Next, click "Add Foreign Shipper" to define the overseas supplier of your capsule filling or sealing machine. Then, under "Add Broker," fill in your freight forwarder or customs broker's details. Staying active in these fields prevents common data-entry delays that could stall your DEA License approval.
(4). Click Create New DEA Form 452
(5). Click to add a new foreign supplier
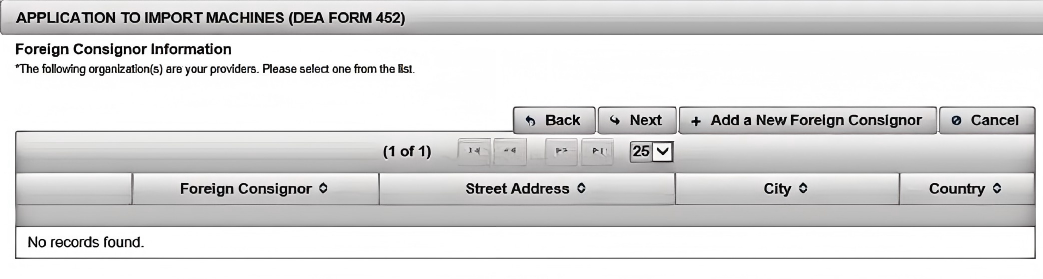
(6). Enter the "Add a Foreign Shipper" fields. Click "Next"

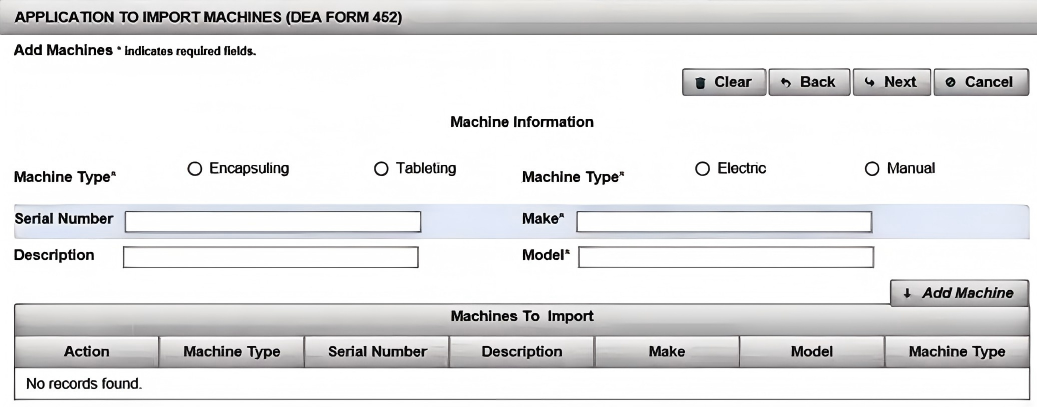
(7). Enter the "Add Machine" fields from the DEA 452 form you want to import. Click "Add Machine". Click "Next"
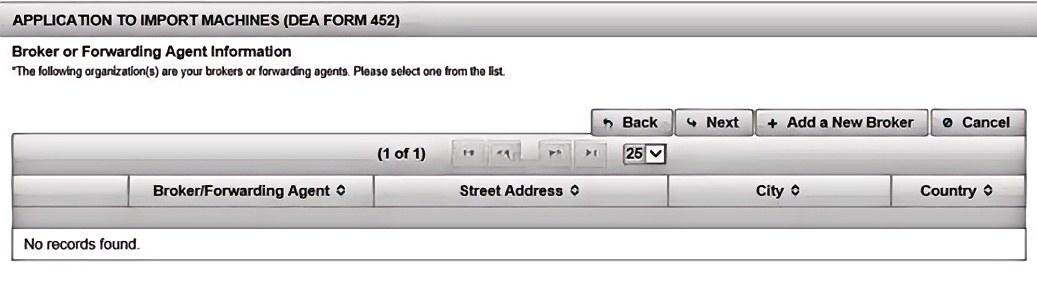
(8). Add New Broker
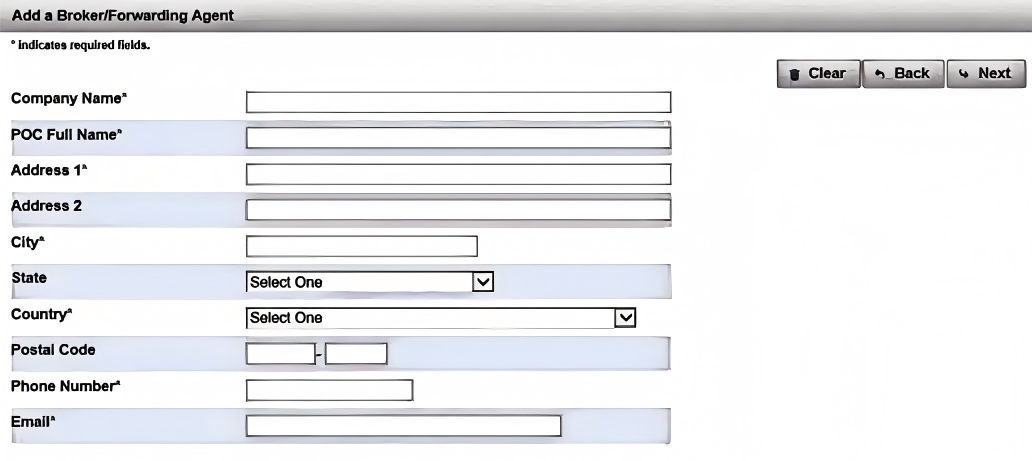
(9). Fill in the fields to add a Broker/Freight Forwarder
Step 3: Provide Shipment & Machine Specifications
In the "Shipment Information" section, supply the shipment date, port of entry, and Estimated Time of Arrival (ETA). Then click "Next," and select your import purpose (e.g., "Commercial," "Scientific," or "Medical"). For each machine, use the "Add Machine" dialogue to specify model numbers, serial numbers, and description (e.g., "Automatic Capsule Filling Machine with Sealing Module"). Accuracy here is crucial: a 2021 report in the International Journal of Drug Policy highlights that detailed equipment specs reduced review cycles by 25%.² By clearly detailing each machine, you help the DEA review your application faster and issue your DEA License without unnecessary queries.
(10). Fill in the Shipping Information fields
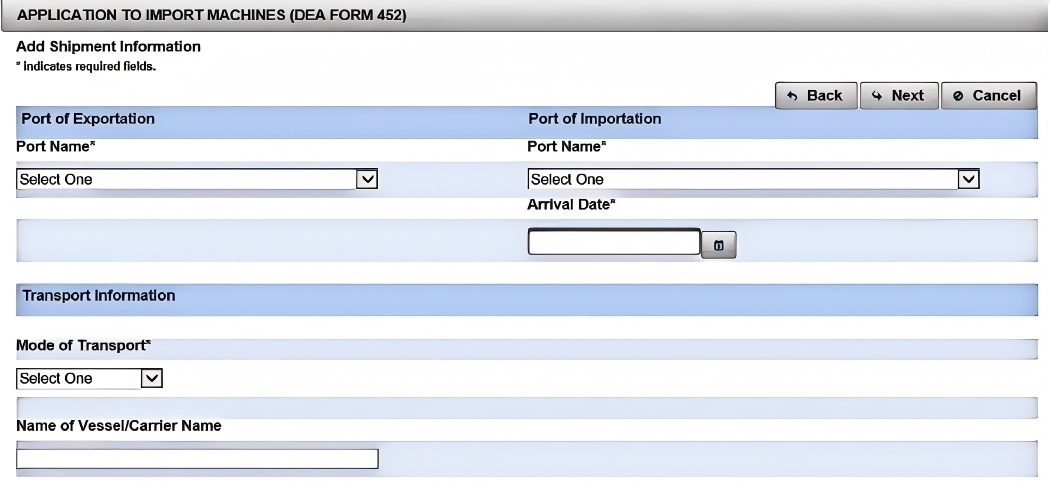
(11). Select the import destination
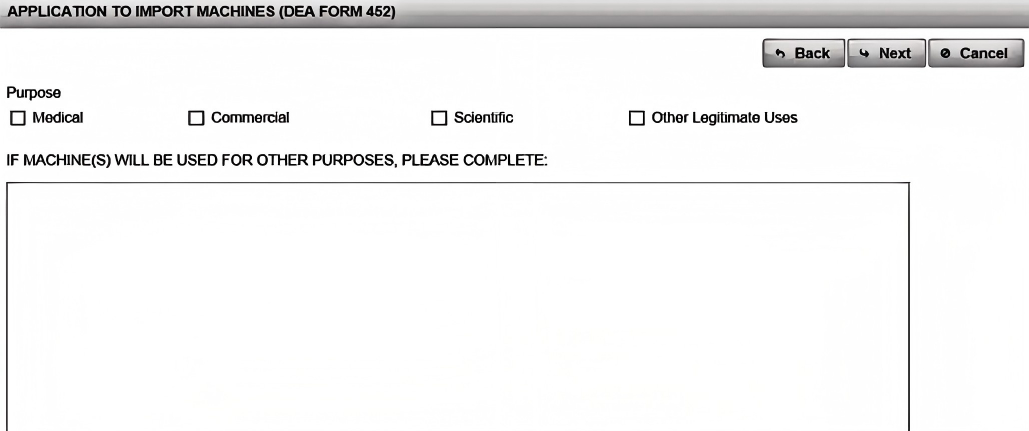
Step 4: Review, Certify & Submit Your DEA Form 452
Before hitting "Submit," carefully review every field. Check the certification box that reads: "I certify that the machines listed are necessary for lawful purposes and the information provided is complete and accurate to the best of my knowledge." Then click "Submit." You'll see a network tracking number---write it down. This number lets you log in daily to monitor when your DEA License transaction number appears next to your Form 452 record in the IMEX RCM system.
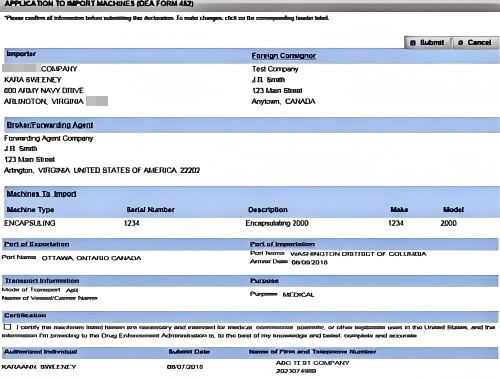
Submit

Write down the network tracking number

Final Tips & Timeline
Most approvals take 10 days to 4 weeks, depending on case complexity. If your application stalls, call the DEA at (571) 362-3279 or email support through the portal. Keep all correspondence professional and concise. Once your DEA License is issued, you can print the completed Form 452 with its official transaction number and proceed with shipping. Proper planning and attention to detail help ensure your machinery---be it a filling machine, labeler, or Vacuum feeder---clears U.S. entry smoothly.
By following this guide, you'll navigate the DEA License application process confidently, minimize delays, and focus on expanding your pharmaceutical-machinery business in the U.S. market.
Buy Cost-effective Tableting and Encapsulating Machines
Ava Duan
Email: sales@grand-packing.com
whatsapp/wechat:86-13787413551

 English
English




























