
On October 23rd, Vivek Ramaswamy made a speech at the BIIS summit. The speech attracted everyone deeply. The beauty of his speech is not how his company succeed in raising more than 3 billion dollars, but that we should put our vision on a broader level. He gave a big prediction about what the biopharma industry will look like in the next 20 years, and he thinks it also applies to China. Maybe, we can call it "Vivek's three predictions".
The following is an excerpt from Vivek Ramaswamy's speech at the 2018 BIIS Summit.
Ⅰ. "Big pharma" will not exist.
From the perspective of investment and return, traditional model estimates its return according to clinical trial quantity, market shares and external mergers and acquisitions, while new model lays more emphasis on research and development. The traditional model will lose efficacy in the future, and the new model is more suitable for the biopharma industry. Therefore, the big pharma will not exist.
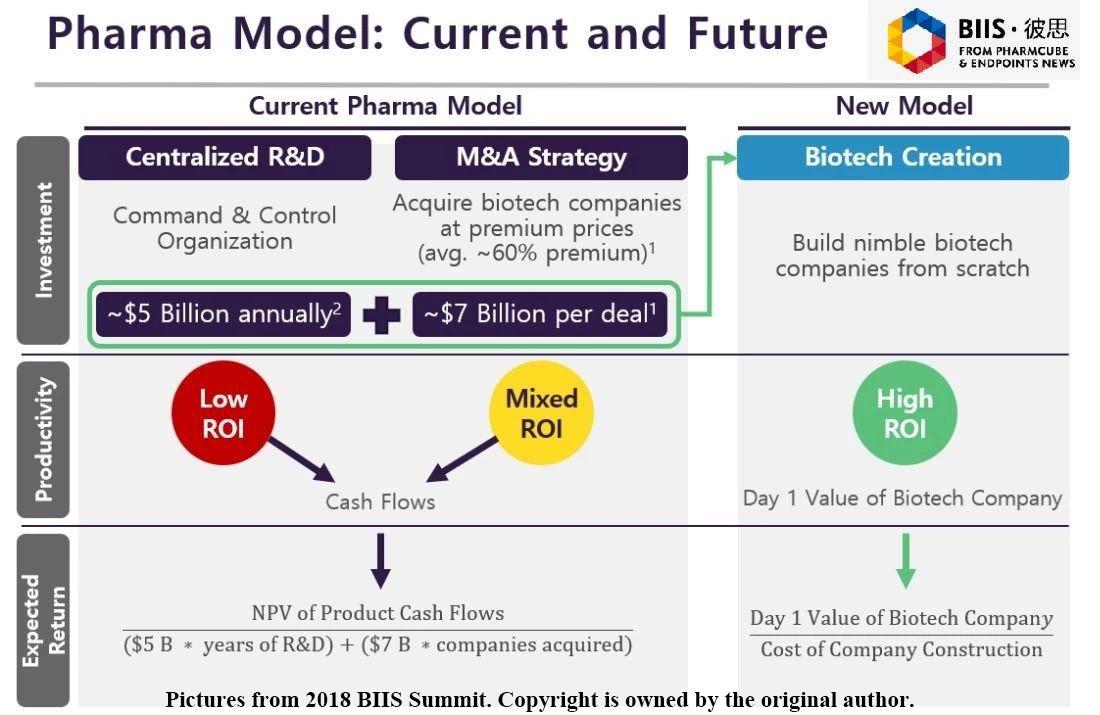
Many companies don't consider seeking profits through different channels until the new drug is made, or, some drug research and development companies are built to be acquired. Whether the company develops new drugs by itself or the company is acquired, it will bring cash flow. For example, suppose a company focuses its energy and capital on developing new drugs and it spends 5 billion dollars developing new drugs every year, then another company needs to pay the premium of average 60% ( like 7 billion dollars) to merger the company. Pure research and development has a low rate of return on investment, as does pure merger and acquisition.
According to my prediction, future pharmas will spend a portion of their fund on research and development, and the rest of the fund is used to purchase mature biotechnology companies. In such way, the fund actually can help a company get new technology quickly and promote the emergence of more new technologies and industrial transformation. The combination of traditional model and new model not only supports research and development of pharmas, but also helps to develop biotechnology companies.
Ⅱ. Pharma companies will generate revenue not just through the sale of drugs.
By 2035, the pharma will achieve considerable revenue by the sale of drugs and some other channels.

From the view of commercialization, commercial sale depends on marketing capacity of company marketing team currently in many countries including America and China. However, future commercialization will no longer rely on pharmaceutical representatives promoting drugs to hospitals (doctors). In the future, the pharmas may promote some tools to help patients get better service from doctors. Patients can utilize these tools to experience products and service from different channels.
It means that future pharmas will not only make profits by selling drugs, but also provide patients with a range of services to improve their compliance rate. This makes it easier to offer patients medical services and develop doctors'work. The pharma also can produce some tools to help more convenient and efficient communication between doctors and patients. By doing so, the marketing of the whole pharmaceutical industry will be easier, and it will be beneficial to pharmas, doctors and patients.
In terms of R&D investment, future drug R&D fund source will not just depend on pharma investment, government funding and venture fund investment, and the fund from the insurance company will be a large part of fund source. I think, there will be many cases that the insurance companies get into medical industry in the future twenty years, such as the health insurance company invests in a new therapy for lower the morbidity and the life insurance company invests in a new therapy for prolong life-span. Instead, they do what they do today like irrelevant security investment.
In the future, not only the pharmas will invest in the biotechnology companies, but also the insurance companies will invest in more biotechnology companies. It is advised that companies which need a staggering amount of money to put into research and development can consider to raise funds from the insurance company.
As to industrial integration, I speculate most pharmas will adopt a brand-new model in the future, similar to Alphabet ―Umbrella Company. The Alphabet adopts the structure of holding company to separate Google research, YouTube, other network subsidies from R&D investment department. It shows us that Google Inc possesses many subsidies and that each subsidy represents a new technology.
Around 2035, we will see that some large-scale pharmas will exist as a unified group and become the parent company of some small biotechnology and health technology companies. Alphabet has existed in such form in Silicon Valley, and Tencent also adopts similar structure in China. But until now, there is no similar structure in the medical industry. Traditional pharmas are facing mounting pressure now. According to my prediction, future pharmas may exist in similar structure, and they need to learn company organization pattern from some Internert companies. Such changes will not only take place in pharmas, but also companies in health industry.
Ⅲ. Biopharma will have eliminated "collective action problems" which currently plague the industry.
Until 2035, the pharmas will have eliminated "collective action problems" in the pharmaceutical industry. For example, the problem of data island will be solved. The database of the medical industry will be connected with other databases, maybe preclinical data, clinical data, or overall databases of medical industry.
Currently, each database nearly exists as an island. They are independent, and they do not connect with each other. The data is not shared between different departments inside a company. In fact, if the data can be shared, patients can be chosen more accurately. The post-marketing data of drugs can help to adjust their operation instruction, reduce clinical side-effect to the maximum extent, and give back early study so as to develop better products.

In America, there are so many independent closed databases. Leftmost databases are some databases for servicing drug distributors, some databases for servicing pharmas, and some databases for research laboratory. The middle databases are these databases for relevant companies governing heath information. Rightmost databases are some application databases, some databases for servicing supplier, some databases for servicing insurance company, and some databases for servicing biomedical company.
Now, these datum only can help their existing customers. In fact, their value is unlimited. If these datum are connected with each other, their value will be infinite. It is a pity that they are closed.
Datavant, a warehouse company in America, has adopted the model. Maybe China also can adopt similar model. Datavant, a software, can respect patients privacy within the company firewall, and meanwhile, it can improve patients' compliance. Though we do not know who they are, we can perceive patients who suffer from the same disease through "de-sensitization".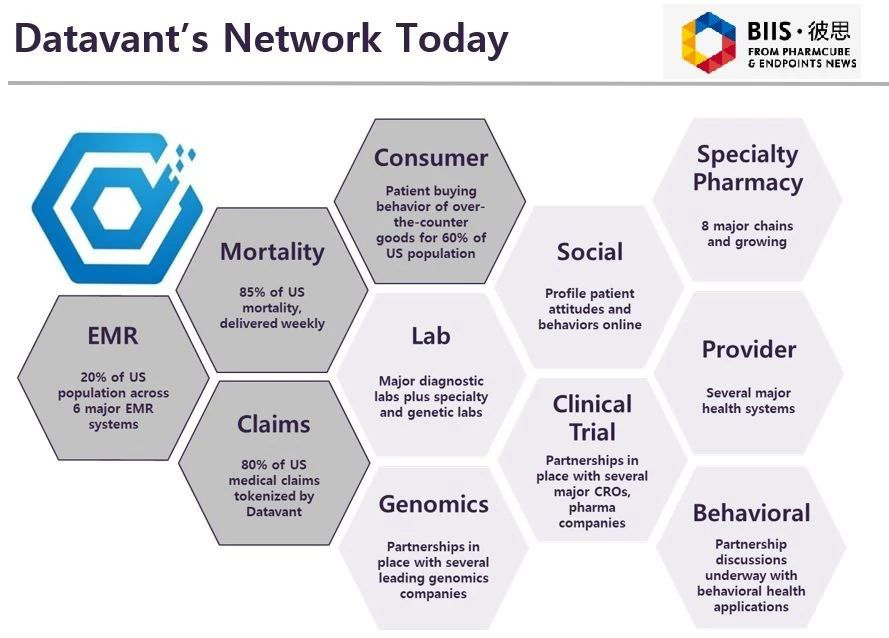
Now, some contents of EMR (Electronic medical record) have been collected by Datavant. At present, the platform can show 80% of weekly death datum, medication administration record before patients die, and claim amount of medical insurance. Some companies can solve the problem of information closure. However, China has not solved this problem. We believe, China will solve information problem gradually.
Another example is about collective action in the medical industry. As a matter of fact, many pharmas around the world are doing repetitive work now. They put their energy and money into the same direction for drug research and development, hoping to find the best in class.
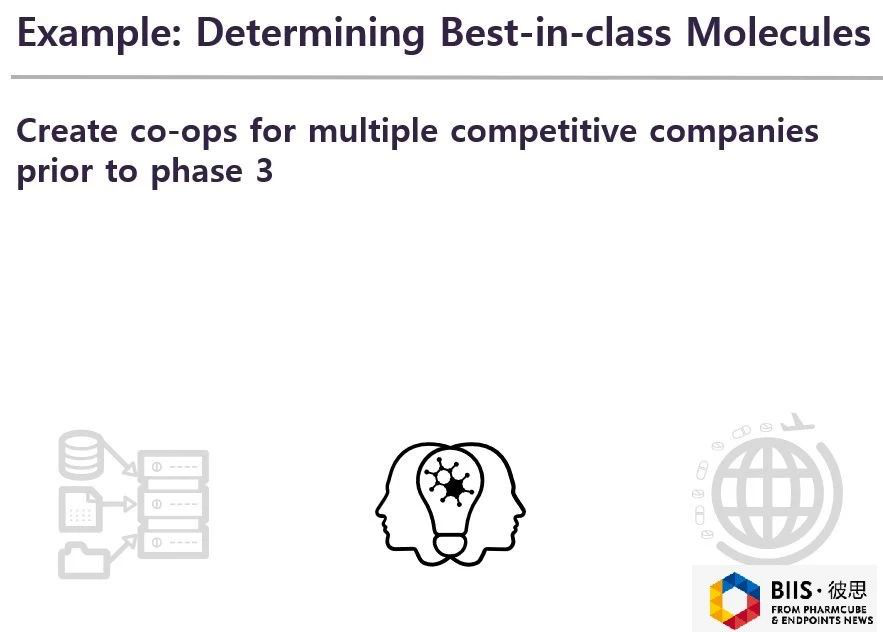
Currently, the new drug development model is that compared with that of placebos (or existing therapies), the clinical data of a new drug has approved if it meets with FDA standard or other standards of regulatory agencies. However, the approval authority may allow many like drugs to come into the market. Doctors hope to study and compare their efficacy, because it is likely that five drugs with same efficacy aim to the same disease. Obviously, this is repetitive work and it is a waste of resource. Some companies make head-to-head comparison of drugs in the clinical stage Ⅲor Ⅳ, hoping to find the best in class eventually.
When a large amount of capital is put into finding the best in class, why do not spend it to other fields or to invest in new technology? Or, is there a technology which can find the best in class in the early? In this way, companies can put their money into investing new molecules or developing new indications.
Repetitive work is a major problem in the pharmaceutical industry now. However, it is clear that this action is beneficial to companies. By doing so, large enterprises can release more resources for better resource distribution. For small and medium enterprises, it costs a lot of money if they conduct repeated clinical experiments in different fields. I heard that more than 100 PD-1/PD-L1 inhibitors are undergoing clinical trials now, and I guess most of these are repetitive study.
As a new generation, we hope to open a new chapter with China. We are willing to build a brand new industrial system from scratch, and we are willing to establish close cooperation with China's enterprises. We think, there are exciting opportunities here.
Vivek Ramaswamy, CEO of Axovant Sciences; President and CEO of Roivant Sciences; Chairman of the Board of Directors of Tekmira Pharmaceuticals Corporation; Co-founder of Campus Venture Network (a technology company acquired in 2009); Bachelor of Biology, Harvard University; Doctor of Law, Yale University.

 English
English







